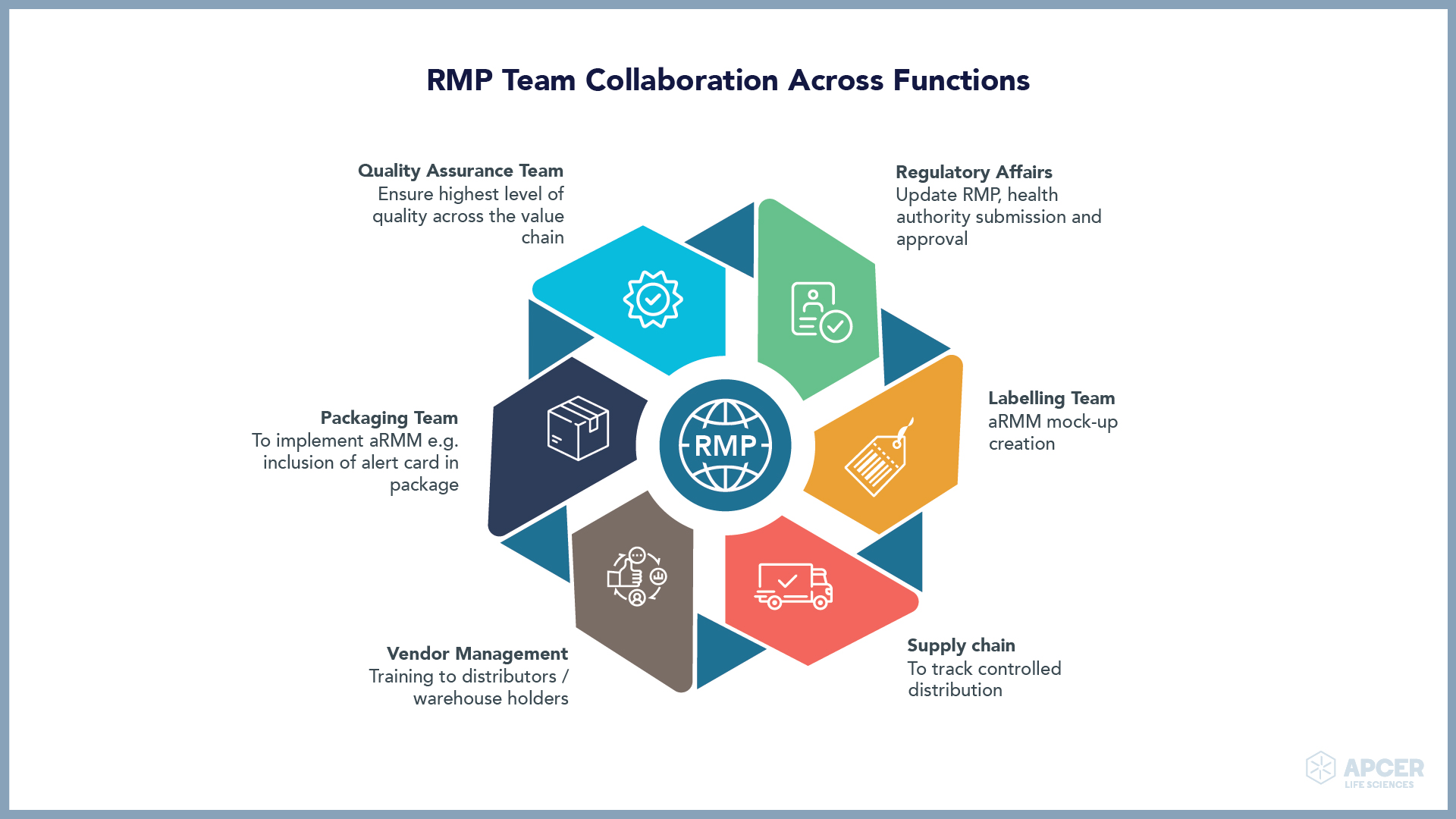
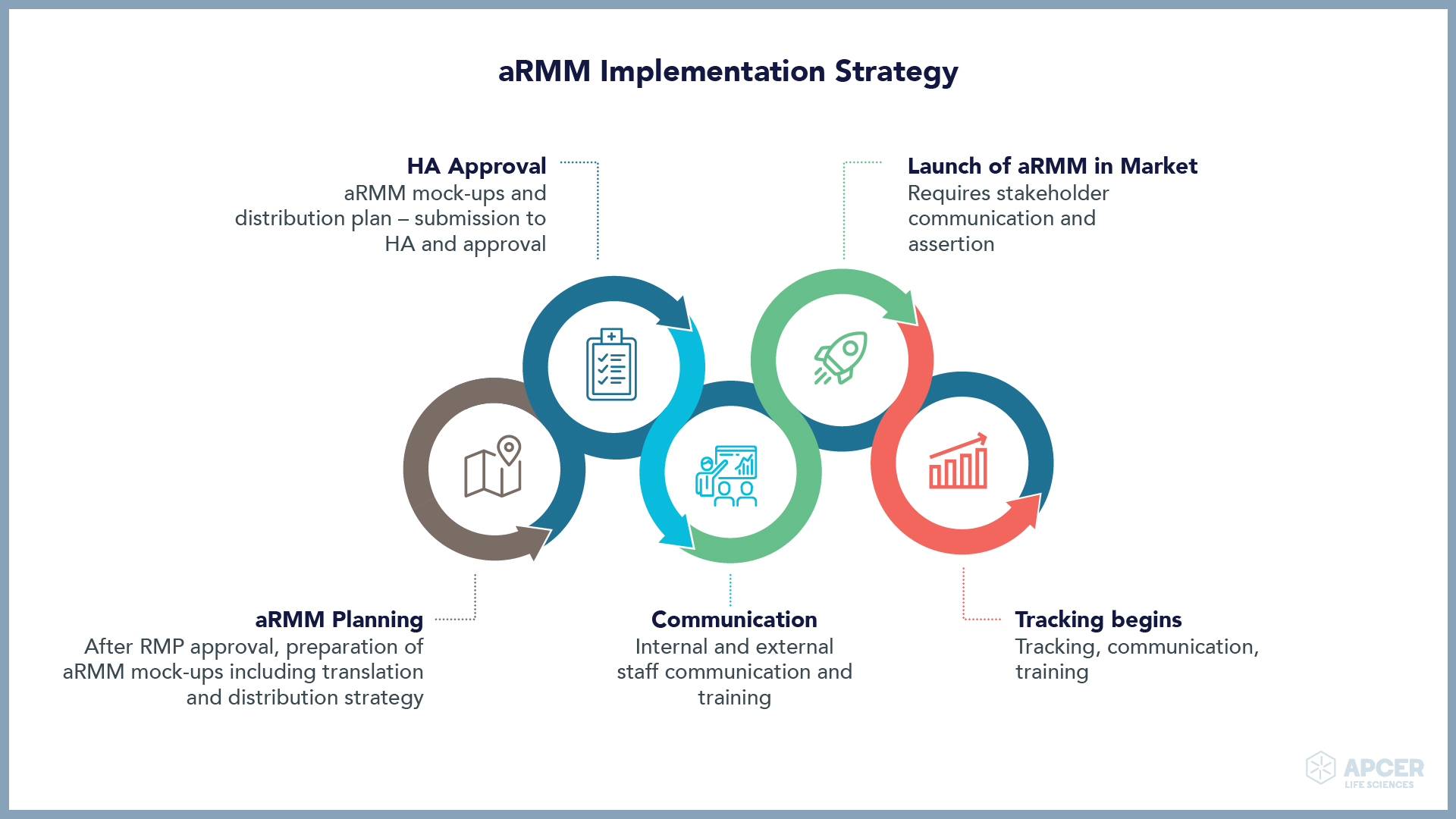
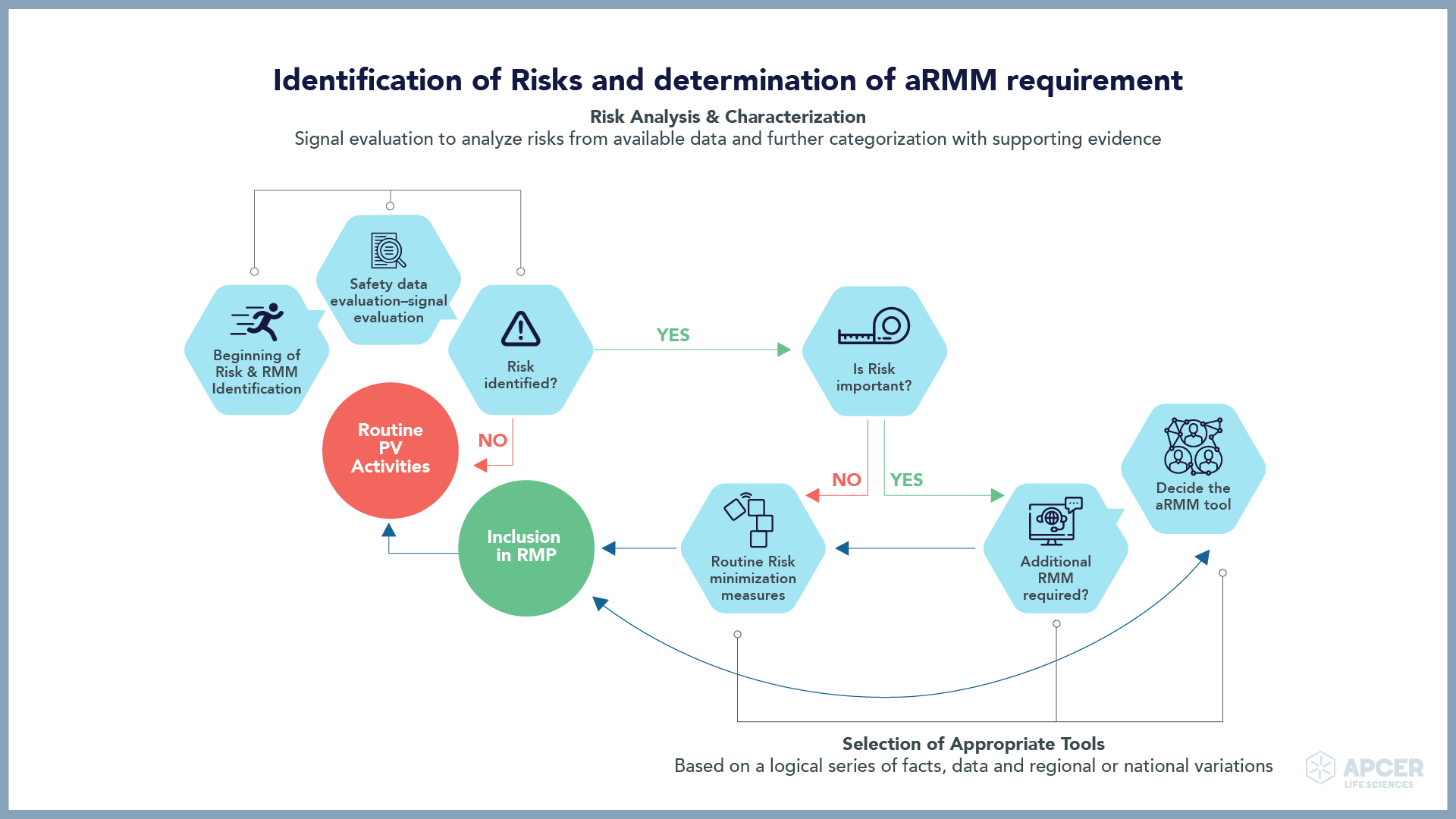
ATMP
March 9, 2021
March 8, 2021
APCER’s Pharmacovigilance (PV) team supported a client’s expansion into new …
March 8, 2021
APCER’s Pharmacovigilance (PV) team helped a biotech pharmaceutical company with …
March 8, 2021
APCER helped a global pharmaceutical company in establishing a collaborative …
March 8, 2021
APCER Life Sciences supported one of the biopharma companies in …
March 8, 2021
Read how our Quality Assurance team helped a pharma company …
March 8, 2021
APCER’s Pharmacovigilance (PV) team provided PV services right from set …
March 8, 2021
We helped a biopharma company to set up end-to-end Quality …
March 8, 2021
APCER Life Sciences helped develop the strategy and provided medical …
March 8, 2021
Our medical monitoring team with experience and expertise in early …
January 12, 2021
Ms. Jeanne Schow, Vice President & US Head of Business …
December 11, 2020
Dr. Vineet Kacker, Managing Director & Global Technical Head talks …