Together for
new horizons
Specialty Areas
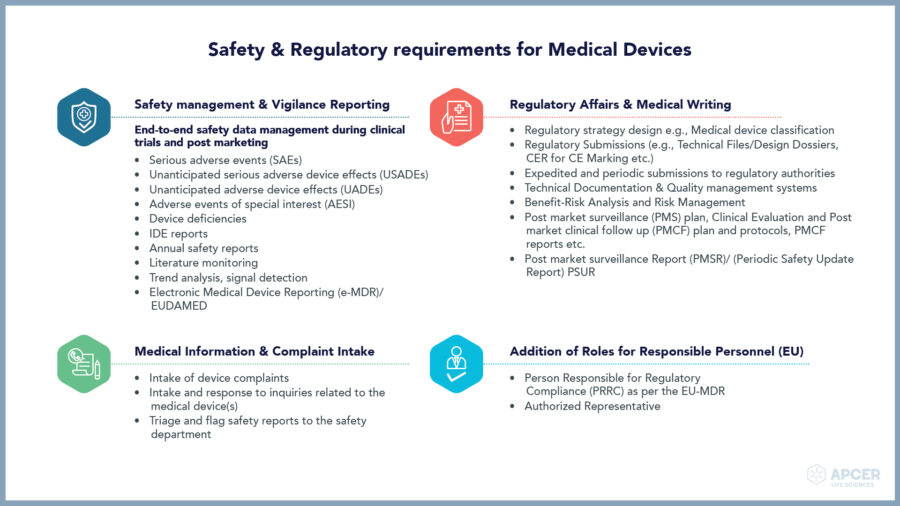
Safety requirements for your advanced therapy, medical device and combination products are getting more stringent. Ensuring the safety and efficacy of these products without impacting the time-to-market requires a Pharmacovigilance partner that can bring strong therapeutic and domain expertise for full life cycle management.
Advanced Therapy Medicinal Products (ATMPs) are gene, tissue or cell based therapies offering groundbreaking treatments for life-threatening or rare diseases.
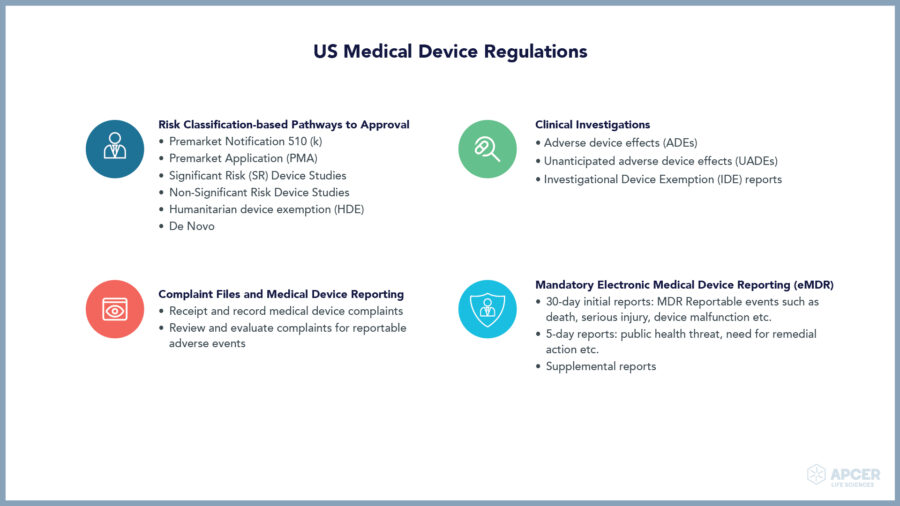
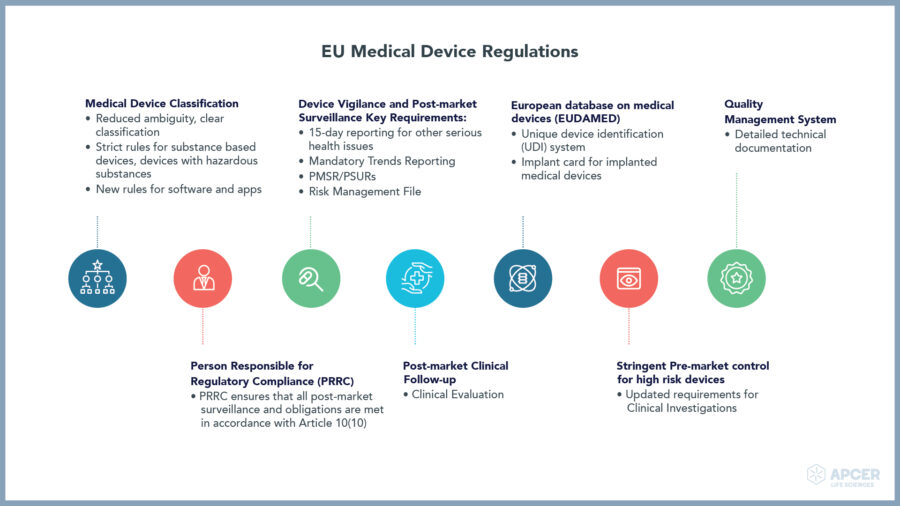
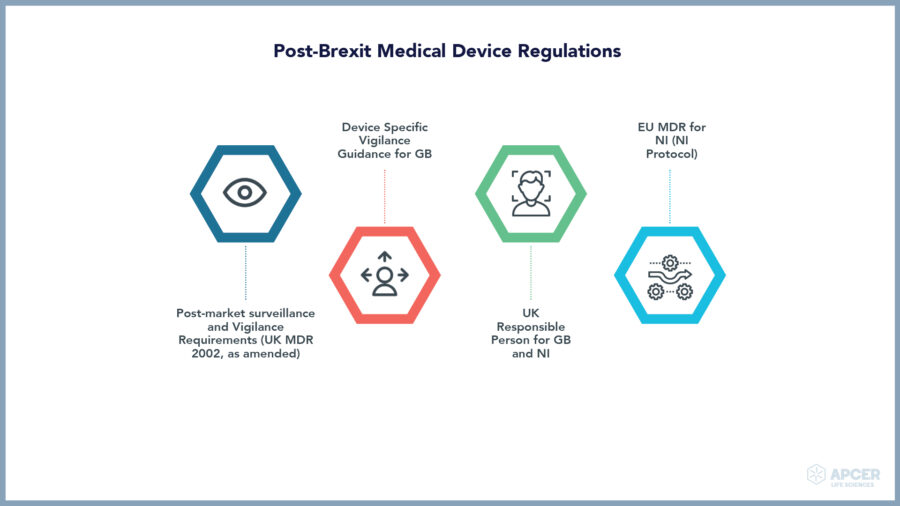
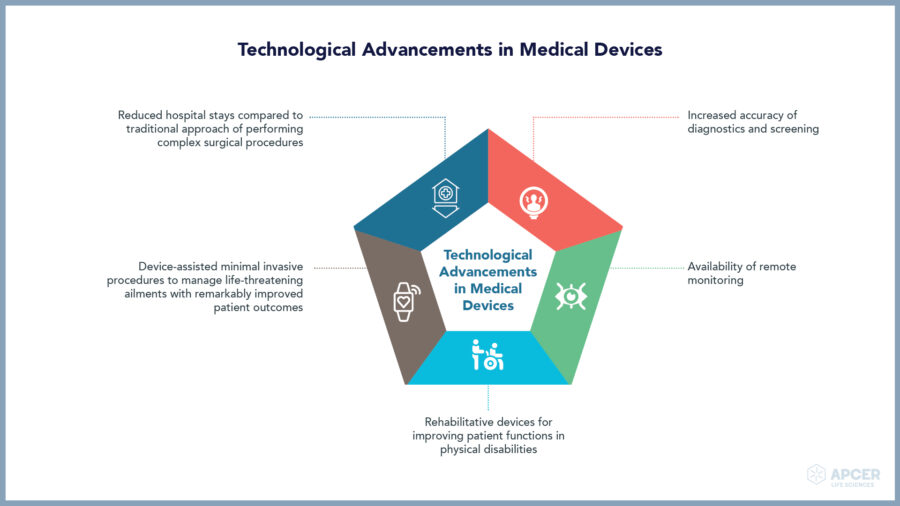
Medical devices play a key role in providing better diagnosis, screening, monitoring, rehabilitation, treatment and care of patients thereby improving the overall quality of life.